Request Your Audit Report Today
Compliance made simple and cost-effective.

Saving time on audit planning is now a reality
Instant access
Dive into the world’s largest Audit Library and explore completed reports at your fingertips.
Effortless savings
Join upcoming planned audits to save time and reduce costs.
Tailored to you
Can’t find the report you need? Request your own audit today. We’ll have it scheduled within 6-8 weeks, cutting compliance costs by up to 70%, all backed by the expertise of our network of 250+ auditors.
Qualifyze Audit Reports benefits
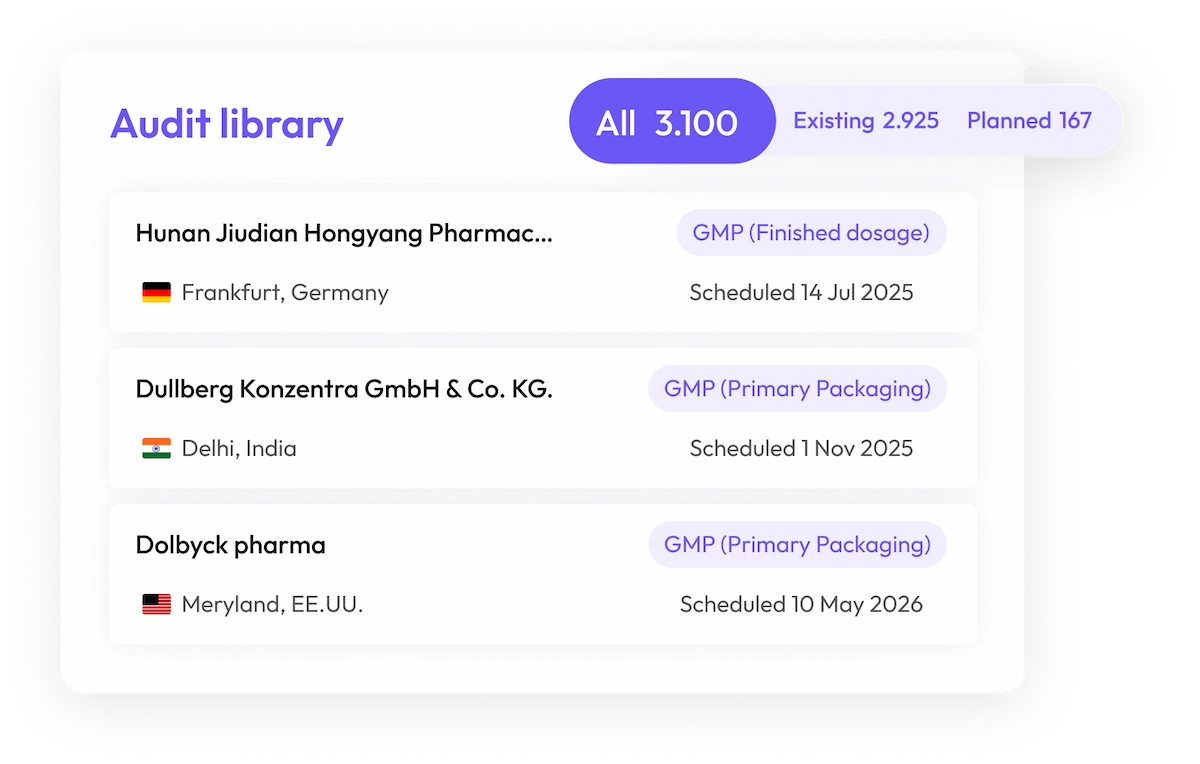
5.000+ audits and counting
Access the largest audit database in the world
Get instant access to our unmatched GxP audit database, featuring over 5.000 audits and counting
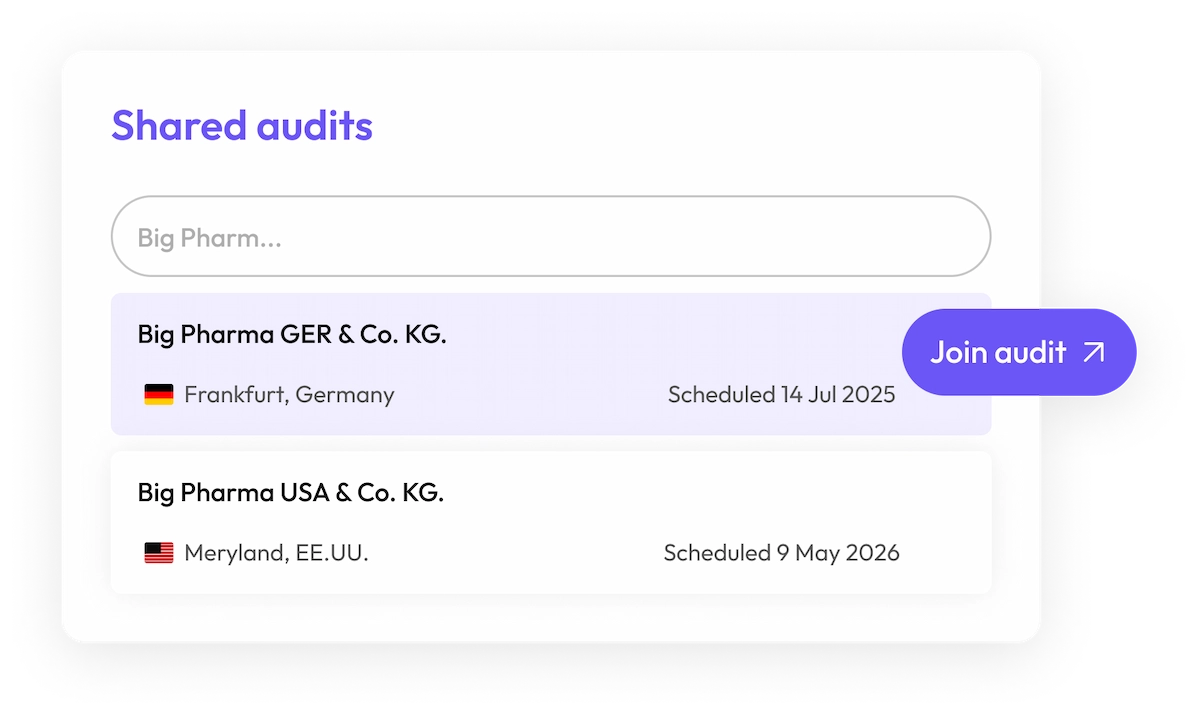
Join scheduled audits
150+ new audits conducted every month
Skip the waiting game. Join your supplier’s next audit or purchase their most recent one.
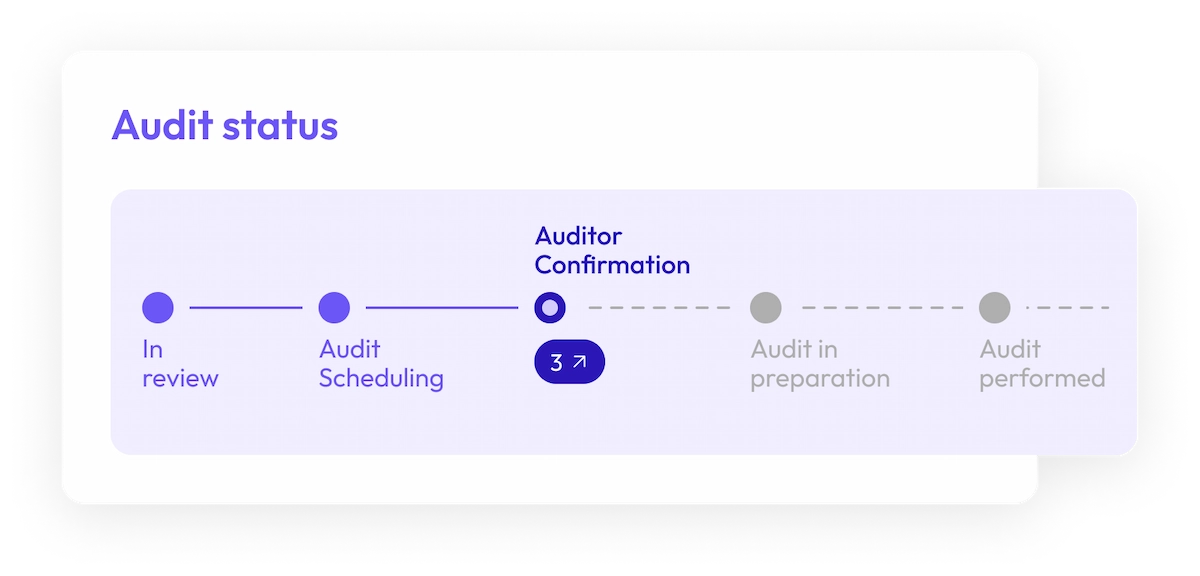
Save time, energy and resources
Improve efficiency and expedite your audit process. From request to delivery, a new audit can take months to finalize. Our audit library lets you spend less time chasing audit dates and dealing with urgent audit requests, and more time to focus on other tasks.
Explore all the audits
Qualifyze is specialized in:
Bespoke audits
Internal audits | Mock inspections | Gap analysis
Upon agreement
Cosmetics
ISO 22716
Packaging materials
Primary and Seconday
ISO 15378 | ISO 9001
Applicable parts of the EU GMP Part 1 when necessary
Other vendors
ISO 9001
Excipients
IPEC, PQG Joint GMP Guide for Pharmaceutical Excipients
Food
IFS/BRC | FSSC/ISO
API (Active Pharmaceutical Ingredients) / Drug substances
ICH Q7, EU GMP Part II
Clinical Trials
Investigational sites | Vendors/Suppliers | Laboratories
ICH GCP and applicable GxP guidances when necessary
ESG
GRI Sustainability Reporting Standards
Software vendors
GAMP 5
Medical Devices
EU MDR (GMP) | ISO 13485
FDF (Finished Dosage Forms) / Drug products
EU GMP Part I (Including its Annexes when necessary)
Transport and/or Distribution (raw materials and FDF)
EU GDP (2015/C/95/01) and/or EU GDP (2013/C/343/01)
Pharmacovigilance
Services | Vendors
EMA GVP and applicable GxP guidances when necessary
Subcontracted Laboratories
Physicochemical testing | Microbiological testing | Bioanalytical testing | Stabilities | …
Applicable GxP guidances
We’ve helped more than 1500 companies stay compliant







Trusted by our clients
”It’s the closest to having an extra member in our team. From alerts to CAPA follow-ups to structured reports, Qualifyze gives us everything we need while making the most out of our resources.”
“We chose to collaborate with Qualifyze, not only for their extensive audit database and fast delivery times but also for the precision and high quality of their reports.”
“Aenova is aware of this responsibility and therefore prefers to work with excellent partners, such as Qualifyze”
“Qualifyze helped us reduce costs and time thanks to their team of experienced auditors.”
“When Qualifyze audited us in 2021, we were impressed by the auditor’s qualification, precision, and profound knowledge. The audit also helped us a lot since it was comparable to an authority audit.”
“We have valued our experience working with Qualifyze on audit reports because of their reliable customer service, collaboration and thoroughness.”
“We chose Qualifyze because of their reliable customer service and the good quality of the audit reports at a fair price. Our requests were handled with flexibility and willingness to compromise.”